Technical partner, Engineering Consulting Office, One of the accreditation offices of the General Authority for Industrial Development Consulting Engineering

Activities
Regulatory Affairs
We have the honor to provide all professional consultations in the field of manufacture of pharmaceuticals, including human and veterinary medicines, disinfectants, Personal care products including Dietary supplements and cosmetic products, medical supplies in addition to medical supplies and apparatus.
Projects execution/Licensing
– Industrial projects: Includes carry out all studies and activities for the execution of industrial projects in the fields of human and veterinary medicines, food supplements, medical supplies, cosmetics, disinfectants, detergents, air fresheners, food, children’s food, athletes, medical food and other pharmaceutical, health and medical products.
Project Inspection
Includes carry out all studies and activities for the inspection of industrial projects in the fields of human and veterinary medicines, food supplements, medical supplies, cosmetics, disinfectants, detergents, air fresheners, food, children’s food, athletes, medical food and other pharmaceutical, health and medical products.
Technical consultations
The office work by his network of in-House technically registered consultants in all fields of activities covered by the office. It also works with its network of freelance and outboard consultants in addition to consultant companies which is bound to office with official sub-sub-contractors contracts.
Training
We help new companies to improve importation/exportation of their products by helping them to register their products in some countries/regions as: MENA region, North America, Europe, USA, Africa, and Arab Gulf.
Preclinical clinical studies
PHARMA ADVISOR works with a first-class network of validated clinical research organisations (CROs) to provide expert experience, skills andknowledge to take pharmaceutical products from concept through to distribution.
International regulatory services
We help new companies to improve importation/exportation of their products by helping them to register their products in some countries/regions as: MENA region, North America, Europe, USA, Africa, and Arab Gulf.
PHARMACOVIGILANCE
Pharmacovigilance consulting is constantly warranted throughout the medicine’s life cycle, starting from the establishment of the development program, continuing through the Marketing Authorisation Application, and later during post marketing periodic reporting to regulatory authorities.

The STAFF
Professional team creates Professional work

Partners
Successful partners means successful projects
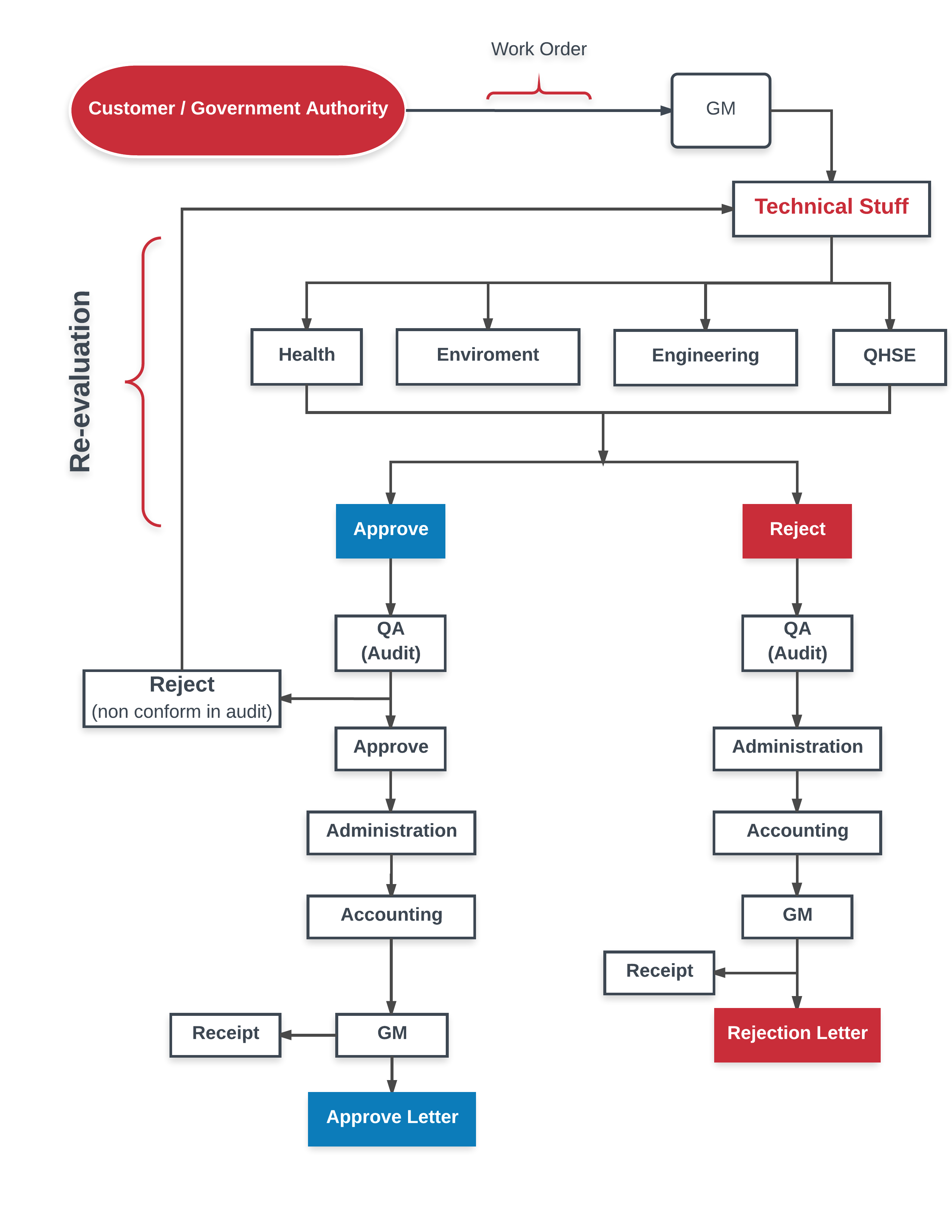
Our Workflow
Recent News
Learn from the top thought leaders in the industry.
Pharmaceutical factory -sterile products, EMA
specifications, UAE The design, construction, start -up operation and licensing of a sterile pharmaceuticals Plant approved by EMA in Free Zone - Egypt - Estimated capital: 75 million dirhams
Dietary supplements factory, EMA specifications, UAE
The feasibility study, design, construction, start -up operation and licensing of a dietary supplements Factory approved by EMA in Free Zone - Dubai - United Arab Emirates - Estimated capital: 20 million dirhams
Pharmaceutical factory, EMA specifications, Tunisia
The feasibility study and the implementation and licensing of a pharmaceutical factory approved by EMA in the Republic of Tunisia - capital estimated - 10 million US dollars
Contact
If we didn’t answer all of your questions, feel free to drop us a line anytime.